The Importance of Ingress Protection Testing for Medical Devices

16 Jan 2025
Ensuring Safety and Performance for Both Healthcare Professionals and Patients
As medical technologies advance and patient needs continue to evolve, manufacturers must prioritize rigorous testing to meet regulatory standards and protect patient health. One of the most crucial types of testing for medical devices is Ingress Protection (IP) testing. IP testing evaluates a device's resistance to dust, water, and other environmental factors.
But why is IP testing so important for medical devices, and what role does it play in ensuring device functionality and patient safety?
What is IP Testing?
IP testing is based on an IP rating system, a widely accepted classification used to define the degree of protection provided by a device's enclosure against the intrusion of foreign objects (like dust or debris) and the entry of liquids (such as water).
This rating is composed of two digits:
- First Digit (Protection against solid objects): Ranges from 0 (no protection) to 6 (dust-tight).
- Second Digit (Protection against liquids): Ranges from 0 (no protection) to 9K (protected against high-pressure, high-temperature water jets).
The higher the numbers, the greater the protection. For example, an IP67-rated device is considered dust-tight (6) and can withstand immersion in water up to a depth of 1 meter for 30 minutes (7).
The Full Range of IP Ratings & IP Testing Required for IEC 60529 Rating
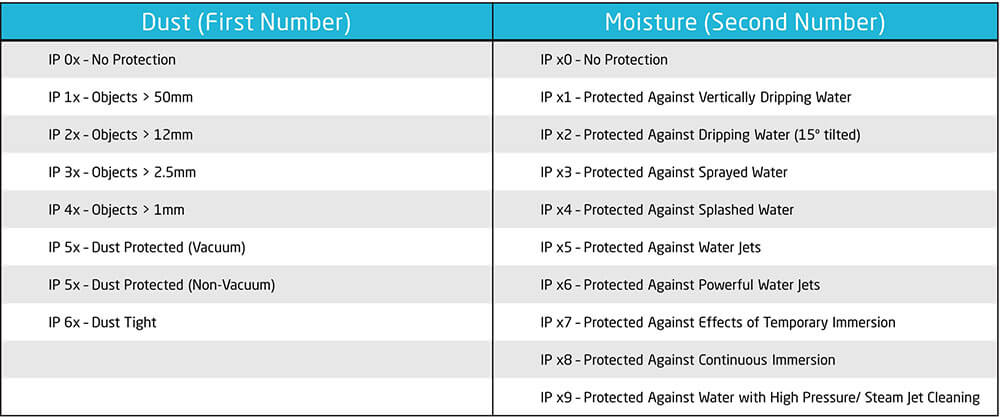
By understanding these different IP levels, manufacturers can tailor their device design and protection to ensure they meet the appropriate standards for the environments in which the devices will be used.
Why is IP Testing Crucial for Medical Devices?
Ensuring patient safety is paramount as medical devices are designed to function in various environments, from hospitals and care facilities to patients' homes. These devices are often exposed to bodily fluids, cleaning solutions, and environmental factors such as moisture and dust. Without adequate protection against these elements, devices can malfunction, pose risks of electrical shock, or even harm patients or users. For instance, a malfunctioning infusion pump could deliver incorrect medication, while water intrusion could short-circuit critical life-supporting equipment.
Compliance with regulatory standards is another critical reason for IP testing. To be marketed and sold globally, medical devices must meet stringent regulatory requirements. IP testing plays a key role in these compliance processes, as regulatory bodies like the Federal Drug Administration (FDA) in the United States and CE Marking in Europe mandate that devices meet specific safety criteria, including resistance to environmental factors.
Protecting device performance is equally important, particularly for medical devices used in critical care settings or home healthcare. Devices exposed to moisture, dust, or contaminants may experience performance degradation or failure if their enclosures are not sufficiently protected. For example, ventilators may encounter moisture during cleaning, and pacemakers face potential dust exposure when implanted. IP testing ensures that devices can withstand such challenges and continue operating at peak performance.
Consumer confidence and demand are also tied to robust IP testing. Medical devices are held to high standards by both regulatory bodies and healthcare professionals, as well as patients. A strong IP rating is a significant selling point, especially for devices used in challenging environments such as outdoor settings, surgeries, or homes. High levels of protection against dust, water, and other contaminants enhance trust and the product's reputation.
Finally, IP testing helps minimize maintenance costs. Devices that fail due to environmental factors can lead to expensive repairs, replacements, and service interruptions. For instance, a device damaged by moisture or dust might result in costly repairs and operational downtime. Proper IP testing enables manufacturers to identify potential weaknesses in the design before the devices reach the market, allowing them to take preventive measures.
IP testing plays a pivotal role in ensuring that products meet safety, performance, and regulatory requirements. By assessing a device's resistance to environmental factors like dust and water, manufacturers can ensure that their products are reliable, safe for use, and market ready. Beyond compliance, IP testing builds consumer confidence, helps mitigate risks, and enhances the overall performance of life-critical medical devices.

